

|
|
|
|
Tables, Data and Formulas for solid fuel heating engineers.  See also: Ringelmann Smoke Chart SOURCES: TSI - CombustionAnalysis Basics http://dl.icdst.org/pdfs/files/0446bcb915db6a23f2c60dbe534ac99d.pdf APPLIANCE PERFORMANCE - SIEGERT'S FORMULA For estimating appliance efficiency from CO2 concentration in flue gas 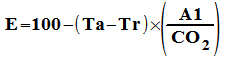 Where: E is Efficiency as a percentage Ta is the temperature of the exiting flue gas, in ºC Tr is the ambient (room) temperature, in ºC CO2 is the percentage CO2 in the exiting flue gas A1 is a fuel factor: Anthracite=0.683, Coke=0.290, Bituminous Coal=0.672, Lignite=1.0, Peat Briquettes=0.7, Dry Wood=0.650 To infer CO2 concentration from known O2 concentration: The 'CO2 max' for a particular fuel is on the Fuel Properties Table - wood = 19.1 The total heat output in kW is then estimated from: 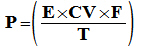 Where: P is the total heat output in kW E is the Efficiency as a percentage F is the fuel used, in kg T is the burn time in hours CV is the potential heat of the fuel in kW per kg, using data on the fuel properties page Boiler output is then calculated from: Where: Pw is the boiler output in kW Cp is the specific heat of water = 4.18 Mw is the water flow rate in kg / hr N is the water temperature rise in Celsius degrees The room heating output of this boiler appliance is then estimated by subtracting the boiler output Pw from the previously calculated total output, P. 'NET' AND 'GROSS' EFFICIENCY The 'net' (higher) efficiency figure applied to solid fuel appliances tested to EN Standards doesn't account for the heat wasted in boiling off the water in the fuel. It is a bit difficult to calculate, but the more accurate 'gross' (lower) efficiency is usually about 0.91 of the the net efficiency for wood fuels and about 0.98 for mineral fuels, so a European wood-burning appliance claiming to be 80% (net) efficient is probably closer to 73% (gross). See: The UK Government’s Standard Assessment Procedure (SAP) for assessing the energy performance of dwellings: http://www.bre.co.uk/filelibrary/SAP/2009/SAP-2009_9-90.pdf CHIMNEY PERFORMANCE (ROUGH) (Hughes' Formula) Chimney pressure: Where: ΔP = The pressure difference (draught) in Pascals Tc = Average chimney flue internal temperature, °C Ta = Ambient (outside) temperature, °C h = The chimney height in metres. OR... Flue draught = Assuming that the molecular mass (i.e., molecular weight) of the flue gas and the external air are equal and that the frictional pressure and heat losses are negligible: where: Q = chimney draught/draft flow rate, m³/s A = cross-sectional area of chimney, m² (assuming it has a constant cross-section) C = discharge coefficient (usually taken to be from 0.65 to 0.70) g = gravitational acceleration, 9.807 m/s² H = height of chimney, m Ti = average temperature inside the chimney, K Te = external air temperature, K. OPEN FIRES Openings for open fireplaces 'The Method of Metres' The maximum area of an open fireplace opening is (Hughes' Formula) Where: A = Fireplace opening area H = Height of chimney in metres F = Flue cross-section area APPLIANCE PERFORMANCE (ROUGH) Appliance output (kW) (rough guess) = volume of firebed (m³) x 400 GAS CONCENTRATION To (approximately) convert between mass per unit volume and ppm (parts per million) OR Where: mg/m3 is milligrams of pollutant per cubic meter of gas ppmv is pollutant concentration, in parts per million by volume T is ambient temperature in Kelvin (temp in °C + 273) 0.08205 is the universal gas constant M is the molecular mass (or molecular weight, in g/mol) of the substance, CO = 28, CO2 = 44, NO2 = 46, CH4 = 16 ENERGY STORAGE IN WATER Water can store about 4181 Joules of heat energy per kg (or litre) of water for every degree (°C) of temperature difference between the water and whatever (usually the room air) it is going to be transferred to. So, given a thermal store of 400 litres capacity at 70°C when the house around it is at 15°C, how many hours heat can it store? The temperature difference is 70-15 = 55°K, multiplied by the capacity of 400, multiplied by 4181 = 91982000 Joules stored. A Joule is a Watt per second and a kW is a thousand watts (per hour), so divide by 3 600 000, and a 400 litre store can hold about 26kW. If the home has a heat requirement of, say, 6 kWh, then the store can provide about 4 hours heat. Note that if the stored temperature was raised to 80°C, the store could hold 30kW, about 5 hours heat. Roughly, it takes 1.16 Watts to heat 1 litre of water by 1 °C, and, typically every 100 litres can hold about 6kWh. ENERGY EQUIVALENTS 1 TOE (ton oil equivalent) = 11 630 kilowatt hour 1 TCE (ton coal equivalent) = 8 141 kilowatt hour 1 gigajoule = 278 kilowatt hour 1 megajoule = 0.278 kilowatt hour 1 gigawatt hour = 1 000 000 kilowatt hour 1 therm = 29.3 kilowatt hour 1 terajoule = 277 777.777 78 kilowatt hour 1 barrel of oil = 1628 kWh 1 ton wood = 5140 kWh SMOKE MASS CONVERSIONS Smoke (particulates) quantity can be expressed in several different ways, none of which is readily converted to the other. The following are approximations based on the stove matrix Where... 1 mg smoke per m³ flue gas = 3.1 mg smoke per kW heat output = 15.7 mg smoke per kg fuel wood = 0.86 mg of smoke per GigaJoule (GJ) heat output 0.0009 mg of smoke per MegaJoule(MJ) heat output AIR FLOW THROUGH ORIFICES Flow of gas through an orifice is given from 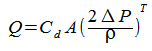 Where: Q = gas flow rate in m³ per second Cd = the 'discharge coefficient', about 0.61 for a hole in a flat plate A = opening area in m² ΔP = pressure difference, in Pascals T = turbulence factor. About 0.5 for a hole in a plate ρ = density of the gas. Air = about 1.2 TYPICAL FLUE AND AIR REQUIREMENTS Open fire fresh air opening to room = c½ minimum flue cross-section Maximum open fire 'face' area = c7 x minimum flue cross-section or, the 'method of metres' - the ratio of room face opening to flue cross-section = the chimney height in metres Closed fire air opening to to room = c550 sq mm per kW Closed fire air opening to firebox = c130 sq mm per kW Low flue draught = c6 Pa = 0•025 ins water Normal flue draught = c12 Pa = 0•05 ins water High flue draught = c17 Pa = 0•07 ins water PROPERTIES OF MATERIALS
FLOW 1 pound/hour = 0•453 592 kilogram/hour 1 kilogram/minute = 60 kilogram/hour 1 (UK) gallon/minute = 0•075 768 litre/second ENERGY 1 calorie [IT] = 4•186 joule 1 calorie [thermochemical] = 0•000 001 162 kilowatt 1 Btu = 0•000 293 071 kilowatt = 1 055 Joule 1 kilowatt = 3 412•14 Btu/hour 1 therm = 100,000 Btu = 29•30 kilowatt hour 1 joule = 1 Watt per second 1 kW/hr = 3,600,000 Joules 1 Watt/hr = 3,600 Joules PRESSURE 1 bar = 100 000 pascal = 100 kPa 1 inch of water [4°C] = 249 pascal 1 millimetre of water [4°C] = 9•81 pascal 1 metre of water [4°C] = 9806 pascal = 9•81 kPa 1 newton/square metre = 1 pascal 1 pound/square inch = 6 894 pascal Standard Atmosphere = 1•013 250 bar THERMAL TRANSMITTANCE Btu/hr,ft²,°F x 5•675 = W/m²,°C R value (thermal resistance) = 1/U-value LENGTH 1 metre = 3•281 feet (ft) = 39•37 inches (in) 1 inch = 25.4 millimetre AREA 1 square foot = 0•09289 m2 1 square yard = 0•09289 m² VOLUME 1 cubic metre (m3) = 1000 litres 1 US gallon = 3•785 litres, 1 Imperial gallon = 4•546 litres MASS 1 kilogram (kg) = 2•205 pounds (lb), 1 pound (lb) = 453•6 grams (g) TEMPERATURE Temperature Celsius (°C) = 0•714 x (°F- 32) Temperature Fahrenheit (°F) = (1•8 x °C) + 32 Kelvin or absolute (°K) = degrees Celsius (°C) + 273•15 1 degree interval (°K or °C) = 1•8 °F interval |
|
MORE FROM Soliftec... Home ● Fuel Costs ● Installation ● Library ● About ● Air Supply ● Blogspot ● Building Rules ● Carbon Monoxide ● CE Marking ● Dictionary ● Efficiency ● Electricity - CHP ● Embodied Energy ● Fascinating Facts ● Fireplace Doctor ● Fuel Properties ● Heat Need ● Heroes ● Legislation ● Manufacturers ● Open Fires ● Ringelmann Scale ● Smog and Smoke Control ● Smoke ● Solid Fuels ● Standards ● Statistics ● Stove History ● Tables, Data and Formulas ● Test Laboratories ● Thatched Roofs ● The Carbon Cycle ● The Chimney Effect ● Wood Fuel ● Email: info@soliftec.com COPYRIGHT and ALL RIGHTS RESERVED: © BUILT WITH WHIMBERRY matrixstats |